Is Human Immortality A Scientific Reality?
If you're alive in 20 years, you may be able to live forever.
By Gary Vey ©2011 Viewzone

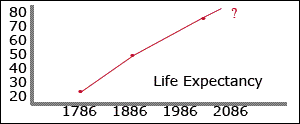 In 1786, average life expectancy was just 24 years. A hundred years later (1886) it doubled to 48. Right now a newborn can expect to live an average of 76 years. With recent discoveries in biology, many scientists predict that life expectancy will continue to triple-digits. In fact, if they are correct, humans shouldn't have to die at all in the future.
In 1786, average life expectancy was just 24 years. A hundred years later (1886) it doubled to 48. Right now a newborn can expect to live an average of 76 years. With recent discoveries in biology, many scientists predict that life expectancy will continue to triple-digits. In fact, if they are correct, humans shouldn't have to die at all in the future."Over half the baby boomers here in America are going to see their hundredth birthday and beyond in excellent health. We're looking at life spans for the baby boomers and the generation after the baby boomers of 120 to 150 years of age." -- Dr. Ronald Klatz of the American Academy of Anti-Aging.
"Anti-aging medicine is not about stretching out the last years of life. It's about stretching out the middle years of life... and actually compressing those last years few years of life so that diseases of aging happen very, very late in the life cycle, just before death, or don't happen at all." -- Dr. Klatz.
Why do we age and die?

The cause of what we call "aging" is now being understood. This new understanding may soon move anti-aging cosmetics and surgery to the ranks of snake oil and Siberian yogurt as life-extension fads -- but not yet. There are a few obstacles that need to be addressed.
Just when you thought that holographic TV and outer space travel were on the future horizon of modern technology, immortality has silently been revealing itself to scientists like Doctor John Langmore [right] of the University of Michigan's Department of Biology.
Dr. Langmore and his group looked inside human cells, at the very essence of human life: the DNA molecule. Specifically, Dr. Langmore looked at the tips of the DNA molecule -- a previously overlooked part of the double-helix molecule -- that contain a kind of chain of repeating pairs of enzymes.
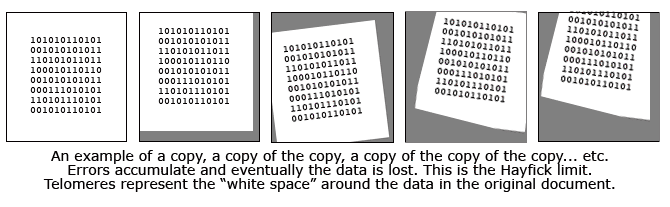
 Called telomeres, these molecular chains have often been compared to the blank leaders on film and recording tape. Indeed, telomeres seem to perform a similar function. During the replication process the spiral DNA molecule must split in half and reassemble a copy of itself. Protecting the vital DNA molecule from being copied out of synch, telomeres provide a kind of buffer zone where mis-alignments (which are inevitable) will not result in any of the important DNA code being lost.
Called telomeres, these molecular chains have often been compared to the blank leaders on film and recording tape. Indeed, telomeres seem to perform a similar function. During the replication process the spiral DNA molecule must split in half and reassemble a copy of itself. Protecting the vital DNA molecule from being copied out of synch, telomeres provide a kind of buffer zone where mis-alignments (which are inevitable) will not result in any of the important DNA code being lost. Perhaps the best analogy I have heard is to compare the telomeres to the white margin surrounding an important type written document. In this analogy, the printed text is the vital DNA code while the white space is the "blank" telomeres. Imagine that this paper is repeatedly slapped on a copy machine, a copy is made, and then that copy is used to make another copy. Each time the paper is subject to errors of alignment and these errors accumulate. After enough copying, it is probable that the white space will diminish and some of the actual text will not be copied. That's what happens inside our cells and it is the reason we get old and die.
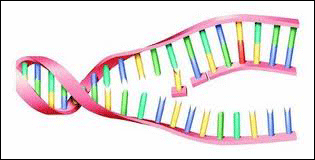 [Right: DNA is a complex molecule that resembles a spiral ladder. When it divides, it splits along the "rungs" then each half of this "ladder" rebuilds the missing half -- voila! -- two DNA molecules. Now the cell can divide. The old cell dies and the new cell continues on.]
[Right: DNA is a complex molecule that resembles a spiral ladder. When it divides, it splits along the "rungs" then each half of this "ladder" rebuilds the missing half -- voila! -- two DNA molecules. Now the cell can divide. The old cell dies and the new cell continues on.] But the procedure is very complex and not perfect. Usually a small portion of the DNA molecule is lost, misaligned and not copied. Since errors are more frequent on the ends of the DNA molecule, this area, the telomere, does not contain any important DNA information and the effect is insignificant.
Telomeres -- programmed to die!
Scientists observe that the length of telomere chains becomes shorter as we grow older. Eventually the telomeres become so short that cell replication produces lethal errors or missing pieces in the DNA sequence, ending the cell's ability to replace itself. This point, when the cell has lost vital DNA code and cannot reproduce, is called the Hayflick limit. It's the measure of how many times a cell can copy itself before it dies.
Some cells in our body have a very high hayflick limit. Cells that line the inside of your mouth and intestines, for example, are constantly being worn away and replaced. Indeed these cells appear to have the ability to regrow telomeres even in aged bodies. Scientists were curious why some cells shut down telomere growth with age, and some do not.
Dr. Langmore used physical, biochemical, and genetic techniques to study the structure and function of telomeres. His group developed a cell-free system to reconstitute functional model telomeres using synthetic DNA, and studied the mechanism by which telomeres normally stabilize chromosomes and how shortening of the telomeres could cause instability.
The protein factors responsible for stabilizing the ends of chromosomes are being identified, cloned, and studied. Electron microscopy is used to directly visualize the structure of the model telomeres. Dr. Langmore's group used new enzymatic assays to determine the structure of telomere DNA in normal and abnormal cells grown in vivo and in vitro, in order to address specific hypotheses about the role of telomeres in aging and cancer. It's exciting research, for sure, and there have been some promising discoveries.
 Scientists have discovered an important enzyme that can turn the telomere production on the DNA molecule "on" and "off." It's called telomerase. Not surprisingly, it seems that as we get older, the amount of telomerase in our cells decreases.
Scientists have discovered an important enzyme that can turn the telomere production on the DNA molecule "on" and "off." It's called telomerase. Not surprisingly, it seems that as we get older, the amount of telomerase in our cells decreases. The Cancer Problem
You might be wondering why biologists don't simply find a way to keep our body's telomeres long. This would prevent replication errors and humans could live indefinitely. The big problem is cancer.
Usually, if a cell makes an error in copying itself, the error will prevent the cell from duplicating itself in the future. So the mistake is limited. But with cancer, cells with errors somehow "turn on" the production of telomerase and make the mutant cell immortal. Now, aberrant cells can reproduce unchecked and outlive normal cells. This is the process that creates tumors.
Since we all have mutant, pre-cancerous cells in our bodies, nature has decided to shut off the telomerase as we age, thus preventing these mutant cells from growing telomeres. It's a kind of programmed death -- a trade off to reduce our lifespan in order to save us from being riddled with tumors. Nevertheless, some pre-cancerous cells manage to re-activate their telomeres and this has caused the research to focus more on blocking telomere production rather than trying to extend it.
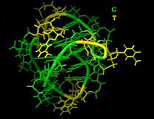
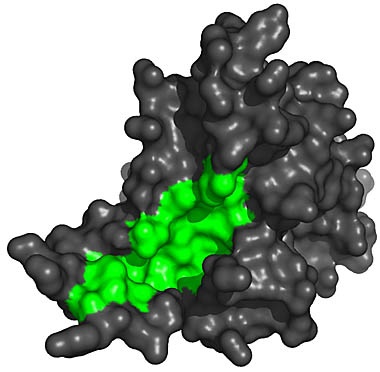
[Right: A 3-d rendering of the telomerase enzyme.] The molecular structure shows an interesting "groove" (show in green) where the enzyme attaches to the end of the DNA molecule.
Ant-cancer researchers believe that by introducing a molecule to block this groove, the telomerase would become unable to attach itself to the DNA and thereby limit the length of telomere production. While this work holds hope for stopping tumor cells from reproducing forever, it does little to extend healthy cells from being rejuvinated. However, if the molecular "blocker" could specifically target only cancerous cells, without blocking telomerase activity in healthy cells, it could be a step towards human life extension if and when a pharmaceutical can be developed that activates telomerase in the human body. [4]
| Interview with Dr. Langmore Viewzone asked Dr. Langmore to give us his thoughts on the role of telomerase, and the possibilities of using it to repair and lengthen telomeres in human cells. His comments follow: Telomeres are special, essential DNA sequences at both ends of each chromosome. Each time chromosomes replicate a small amount of the DNA at both ends is lost, by an uncertain mechanism. Because human telomeres shorten at a much faster rate than many lower organisms, we speculate that this telomere shortening probably has a beneficial effect for humans, namely mortality. The telomere hypothesis of aging postulates that as the telomeres naturally shorten during the lifetime of an individual, a signal or set of signals is given to the cells to cause the cells to cease growing (senesce). At birth, human telomeres are about 10,000 base pairs long, but by 100 years of age this has been reduced to about 5,000 base pairs. ViewZone: How is mortality in non-germ line cells a beneficial effect? Dr. Langmore: The telomere hypothesis of cancer is that the function of telomere shortening is to cause cells that have lost normal control over growth to senesce (i.e. stop growing) before being able to replicate enough times to become a tumor, thus decreasing the frequency of cancer. Immortal cells like cancer have an unfair advantage over normal human cells which are designed to senesce. But nature seems to have planned this human telomere shortening perhaps to prolong life by hindering the otherwise unchecked growth of non-immortal or benign tumors. Malignant, or immortal tumors can simply outlive the rest of the organism. Malignant cancer cells are being studied because they appear to have altered the shortening of telomeres by turning "on" the telomerase. Thus it appears that some cancers and aging are both connected with the biology of telomeres. It is possible that increasing telomerase activity in normal cells might stop the biological clock of aging, yet the side effect of this intervention might be an increase in the rate of cancer. Further understanding and refinement in the telomere hypothesis might lead to a way to slow the aging process and prevent or arrest cancer. However telomeres function, they are an integral part in the very complex process of cell growth, involving many other factors as well. Telomerase might be the Achilles Heal of aging and cancer, but as our understanding of factors that interact with telomerase, factors that are responsible for telomere shortening in the first place, and non-telomerase mechanisms for increasing the length of telomeres, we might find that one of these factors is more easily manipulated to slow aging or prevent cancer. Also there are additional factors that affect aging and cancer, which might prove in the end to be more important than telomeres and telomerase. ViewZone: Are telomeres unique to individual DNA? If so, does this preclude any universal treatment for aging? Dr. Langmore: Different individuals have telomeres with exactly the same DNA sequence but of different lengths. It is too early to say whether there is any relationship between telomere length in an individual and his or her life expectancy, or whether a treatment that would artificially lengthen telomeres would arrest (or reverse) the aging process. One problem is that even in one individual the telomeres of different chromosomes have very different lengths. Therefore an individual might have on average long telomeres; but, he might have one chromosome with a very short telomere that could affect cell growth. ViewZone: In the work of Shay and Wright (see below), increased telomere length was positively associated with telomerase. How significant is this? Dr. Langmore: Shay, Wright and all their many collaborators stimulated telomerase activity in normal cells. This was expected to 1) Increase the length of telomeres and 2) Prolong the lifetime of the cells in tissue culture. The treatment did both, in perfect agreement with the telomere hypothesis of aging. ViewZone: How much was cell lifetime prolonged due to this treatment that reactivated telomerase? Dr. Langmore: The increased proliferation of the cells was perhaps equivalent to hundreds of years of human life. Dr. Langmore received his Ph.D. degree from the University of Chicago in 1975. He has held postdoctoral fellowships at the Laboratory of Molecular Biology in Cambridge and at the University of Basel. |
This structure may help to conceal the end of the molecule from DNA damage surveillance mechanisms and guard against recognition of the chromosome terminus as a double-strand break. [11]
More links to cancer
In the March 15 issue of the European Molecular Biology Organization (EMBO) Journal, Dr. Jerry Shay and Dr. Woodring Wright, both professors of cell biology and neuroscience at UT Southwestern Medical Center at Dallas, report manipulating the length of telomeres to alter the life span of human cells. Shay and Wright are the first to report this important finding. They received an Allied-Signal Award for Research on Aging to explore this line of research last year.
 "By lengthening the telomere, we were able to extend the life of the cell hybrids," Wright explained. "This study is strong evidence that telomere length is the clock that counts cell divisions."
"By lengthening the telomere, we were able to extend the life of the cell hybrids," Wright explained. "This study is strong evidence that telomere length is the clock that counts cell divisions." "The expression of the enzyme telomerase maintains stable telomere length. Telomerase is not detected in normal cells and telomeres shorten and then the cells stop dividing and enter a phase called cellular senescence."
Shay and Wright have shown in earlier studies that telomeres maintain their length in almost all human cancer cell lines. This correlated with inappropriate expression of telomerase and as a consequence allowed the cell to become "immortal." Cell immortality is a critical and perhaps rate-limiting step for almost all cancers to progress. Previous work by the UT Southwestern investigators showed that in a special group of advanced pediatric cancers the lack of telomerase activity correlated with critically shortened telomeres and cancer remission.
Naturally, the exploration of this enzyme is now the focus of much investigation, but for now the research is aimed at understanding how to turn telomeres "off" to limit the spread of "immortal" cancer cells.
Abnormally high levels of telomerase have been found in cancerous breast cells and have been evident in many kinds of tumors.[1]
Consequently, an idea gaining momentum is that the ability to measure and perhaps alter telomere length and/or telomerase activity may give physicians new diagnostic and treatment tools for managing the care of patients with cancer.
Shay and Wright tried to alter already-immortal cells by attempting to inhibit telomerase activity and cause telomeres to shorten. "Unexpectedly, we found the opposite result. Rather than inhibiting telomerase, our treatment caused the immortal cells to develop longer telomeres," Shay explained. "Although we were surprised with the result, we now know there is a causal relationship between telomere length and the proliferate capacity of cells.
"Essentially, we combined the tumor cells containing experimentally elongated telomeres with normal cells and extended the life span of those cell hybrids compared to similar hybrids using cells without experimentally elongated telomeres."
Shay and Wright said the mechanism that causes telomeres to lengthen is still unclear. However, Shay said, "Our observations increase confidence in the hypothesis that immortal cells and reactivated telomerase are essential components of human tumors. Ultimately, we may be able to regulate tumor cells by inhibiting telomerase activity."
The potential implications for research on human aging also are significant. "It is still speculative, but understanding the role of telomere shortening in cell aging may give us the information we need to increase the life span of an organism," Wright said. (News Releases from UT Southwestern)
A New Player: Progeren?

Scientists now believe that the protein called progerin, causing rapid aging symptons in progeria victims [Right], is the same protein that causes our own more standard aging.
Progerin stems from a gene mutation that shows up in abundance in the disease but in more manageable quantities in all of us. It causes extreme premature aging.
"When the telomeres become too short and frayed, this triggers the production of progerin, signaling to the body that the cell is at the end of its useful life." -- Dr. Francis Collins, director of the National Institutes of Health
Rust Never Sleeps
DNA damage occurs continuously in living cells. While most of this damage is repaired, some accumulates. The DNA Polymerases and other repair mechanisms cannot keep up with defects as fast as they are produced. In particular,DNA damage accumulates in non-dividing cells of mammals [13], but all cells eventually need to reproduce and let their "other half" carry on.
Most damage comes in the form of oxidative damage -- the same "rusting" process that oxidizes irong. Arteriosclerosis and heart disease are the results of this type of damage to the cells lining the blood vessels.
Without the ability to duplicate and repair itself, the cell "rusts" to death. In this way we gradually lose members of our youth -- one cell at a time. We wear out and become a mortal.
Is Long Life Inherited?
If your family has a history of living to an old age, it is likely that your telomeres are longer and therefore protect the critical DNA information when your cells make copies of themselves.
In a general population, the people who live longer usually have more offspring. So it follows that the number of people having longer telomeres would increase. But there's the cancer problem. The immortal cancerous cells that we all produce outlive normal cells and eventually express themselves in tumors, shortening life. These two opposing consequences of long telomeres, positive and negative, are balanced by natural selection. The compromise is currently programmed for an average life expectancy of 76 years.
Environmental Factors?
Our cells are bombarded by environmental poisons that can cause the genetic codes to break, regardless of the telomere length. The more toxic the environment is, the greater the chance for "broken" cells to reproduce themselves and the more beneficial it would be to have shorter telomeres to limit this kind of mutation.
What would happen if a population was forced to have offspring at a very young age, eliminating the impact of a toxic environment? This is exactly what happens to laboratory rats and mice who are artificially bred. Their breeding environment is usually free of toxins and highly controlled, thus favoring the expression of longer telomeres. This fact is important because rats and mice are traditionally used to test for toxicity of products in humans. In short: the rats may be too healthy to be our experimental surrogate.
Beware of Lab Rat Inferences

Bret S. Weinstein & Deborah Ciszek [University of Michigan] observed that captive-rodent breeding protocols, designed to increase reproductive output, simultaneously exert strong selection against reproductive senescence (old age) and virtually eliminate selection that would otherwise favor tumor suppression (i.e. having shorter telomeres). This appears to have greatly elongated the telomeres of laboratory mice. With their telomeric failsafe effectively disabled by forced breeding techniques, these animals are unreliable models of normal senescence and tumor formation. Safety tests employing these animals likely overestimate cancer risks and underestimate tissue damage and consequent accelerated aging.
In short: lab rats are bred in an artificial environment that does not allow for the changes of natural selection and so they have become more prone to cancer and artificially immune to the effects of premature aging. As such, their reaction to pathogens should not necessarily be relied upon to indicate human reactions to such things as food additives, radiation and environmental contaminants. This is a serious problem that has, so far, received little acknowledgement. While we effectively test products for causing cancer we risk the mistake of permitting products that can make us age faster.
Children of young mothers live longer
It has also been noted that children of young mothers seem to live longer than children of older mothers. This is because all of the eggs of a female are produced while she is still in her mother's womb. She is born with all of the egg cells that she will ever have. The telomeres of the early produced eggs are very long but become shortened as more are made. So a woman has a finite number of eggs, some with long telomeres and some with shorter telomeres. After birth, when the female reaches puberty, these long-telomere egg cells are the first to be released by the ovaries, and the first to become potential embryos. Thus, having children as near to puberty as possible increases the chance that the offspring will inherit long telomeres. It also explains why having children later in life increases the chance for birth defects or miscarriages. This is precisely what is happening to the laboratory rat and mice populations, who are forced to breed early, and explains why these lab animals have unusually long telomeres compared to animals in the general "wild" population.
It is possible to become immortal and younger
Many have wondered what benefit an older person would have from an immortality medicine. Would they have to be old forever? Thankfully, no. Scientists have already reversed aging, transforming the slow and feeble into the quick and virile.
Harvard scientists reverse the ageing process in mice
Harvard scientists were surprised that they saw a dramatic reversal, not just a slowing down, of the ageing in mice. Now they believe they might be able to regenerate human organs.
Laboratory mouse in a scientist's hand In mice, reactivating the enzyme telomerase led to the repair of damaged tissues and reversed the signs of ageing.
Scientists claim to be a step closer to reversing the ageing process after rejuvenating worn out organs in elderly mice. The experimental treatment developed by researchers at the Dana-Farber Cancer Institute, Harvard Medical School, turned weak and feeble old mice into healthy animals by regenerating their aged bodies.
The surprise recovery of the animals has raised hopes among scientists that it may be possible to achieve a similar feat in humans -- or at least to slow down the ageing process.
An anti-ageing therapy could have a dramatic impact on public health by reducing the burden of age-related health problems, such as dementia, stroke and heart disease, and prolonging the quality of life for an increasingly aged population.
"What we saw in these animals was not a slowing down or stabilisation of the ageing process. We saw a dramatic reversal – and that was unexpected," said Ronald DePinho, who led the study, which was published in the journal Nature.
Conclusions
So for humans to extend life we must do two things: first, eliminate the toxins in our environment that rust the cells. Remember the hayflick limit. A toxic environment can run through those allotted duplications at an accelerated rate.
Next we wait for a scientific breakthrough -- perhaps some pharmaceutical that will re-activate telomere production in healthy cells only. Understanding and controlling telomeres in healthy and cancerous cells will lead to a cure or prevention of cancer. If mortality still eludes us, at least that will greatly extend our lives.
The twenty-first century may well be the era in which humans learn the secrets of life extension, but it may also be a time to be reminded of the many dangers inherent in exploring and exploiting these god-like abilities. Environmental pollution, overpopulation, food shortages, and the concentration of wealth by the few are all factors that pre-destine human immortality to be a highly controversial issue.
From every tree in the garden did he grant them to eat, save but one. And that tree, in the center of the garden, was called the tree of life. And the Snake said to Eve, "Eat of this fruit and you will become as God and never die.-- Genesis 3:1
[1] Steven E. Artandi, Dept of Hematology, Cancer Biology Program, Stanford University School of Medicine, Trends in Molecular Medicine, Volume 8, Issue 1, 1 January 2002, Pages 44-47
[2] Telomere elongation in immortal human cells without detectable telomerase activity, T M Bryan, A Englezou, J Gupta, S Bacchetti, and R R Reddel Cancer Research Group, Children's Medical Research Institute, Westmead, Sydney, NSW, Australia.
[3] Ohyashiki K, Ohyashiki J, Iwama H, Hayashi S, Shay J, Toyama K.,Telomerase reactivation in leukemia cells, Int J Oncol. , 1996 Mar;8(3):417-21.
[4] Thanks to Thomas R. Cech, Steven A. Jacobs and Elaine R. Podell of the Howard Hughes Medical Institute, University of Colorado at Boulder.
[5] Motonobu Katoh, Misaki Katoh, Michihiro Kameyama, Hiroyuki Kugoh, Motoyuki Shimizu, Mitsuo Oshimura, A repressor function for telomerase activity in telomerase-negative immortal cells, Molecular Carcinogenesis, Volume 21, Issue 1, pages 17–25, January 1998
[6] T M Bryan, A Englezou, J Gupta, S Bacchetti, and R R Reddel, Telomere elongation in immortal human cells without detectable telomerase activity, EMBO J. ,1995 September 1; 14(17): 4240–4248.
[7] Steven E. Artandi, Telomere shortening and cell fates in mouse models of neoplasia, Trends in Molecular Medicine,Volume 8, Issue 1, 1 January 2002, Pages 44-47
[8] Calvin B Harley and Bryant Villeponteau, Telomeres and telomerase in aging and cancer, Current Opinion in Genetics & Development, Volume 5, Issue 2, April 1995, Pages 249-255
[9] Fajkus et al., 1996; Fitzgerald et al., 1996; Heller et al., 1996
[10] http://www.frontsidebus.net/2011/06/15/slow-down-new-research-suggests-a-treatment-for-aging/
[11] The Plant Cell, Vol. 16, 794–803, April 2004, www.plantcell.org 2004 American Society of Plant Biologists, HISTORICAL PERSPECTIVE ESSAY: Plant Telomere Biology
Additional Reading & Comments
Harvard scientists reverse the ageing process in mice -- now for humans
Harvard scientists were surprised that they saw a dramatic reversal, not just a slowing down, of the ageing in mice. Now they believe they might be able to regenerate human organs
Laboratory mouse in a scientist's hand In mice, reactivating the enzyme telomerase led to the repair of damaged tissues and reversed the signs of ageing.
Scientists claim to be a step closer to reversing the ageing process after rejuvenating worn out organs in elderly mice. The experimental treatment developed by researchers at the Dana-Farber Cancer Institute, Harvard Medical School, turned weak and feeble old mice into healthy animals by regenerating their aged bodies.
The surprise recovery of the animals has raised hopes among scientists that it may be possible to achieve a similar feat in humans -- or at least to slow down the ageing process.
An anti-ageing therapy could have a dramatic impact on public health by reducing the burden of age-related health problems, such as dementia, stroke and heart disease, and prolonging the quality of life for an increasingly aged population.
"What we saw in these animals was not a slowing down or stabilisation of the ageing process. We saw a dramatic reversal – and that was unexpected," said Ronald DePinho, who led the study, which was published in the journal Nature.
"This could lead to strategies that enhance the regenerative potential of organs as individuals age and so increase their quality of life. Whether it serves to increase longevity is a question we are not yet in a position to answer."
The ageing process is poorly understood, but scientists know it is caused by many factors. Highly reactive particles called free radicals are made naturally in the body and cause damage to cells, while smoking, ultraviolet light and other environmental factors contribute to ageing.
The Harvard group focused on a process called telomere shortening. Most cells in the body contain 23 pairs of chromosomes, which carry our DNA. At the ends of each chromosome is a protective cap called a telomere. Each time a cell divides, the telomeres are snipped shorter, until eventually they stop working and the cell dies or goes into a suspended state called "senescence". The process is behind much of the wear and tear associated with ageing.
At Harvard, they bred genetically manipulated mice that lacked an enzyme called telomerase that stops telomeres getting shorter. Without the enzyme, the mice aged prematurely and suffered ailments, including a poor sense of smell, smaller brain size, infertility and damaged intestines and spleens. But when DePinho gave the mice injections to reactivate the enzyme, it repaired the damaged tissues and reversed the signs of ageing.
"These were severely aged animals, but after a month of treatment they showed a substantial restoration, including the growth of new neurons in their brains," said DePinho.
Repeating the trick in humans will be more difficult. Mice make telomerase throughout their lives, but the enzyme is switched off in adult humans, an evolutionary compromise that stops cells growing out of control and turning into cancer. Raising levels of telomerase in people might slow the ageing process, but it makes the risk of cancer soar.
DePinho said the treatment might be safe in humans if it were given periodically and only to younger people who do not have tiny clumps of cancer cells already living, unnoticed, in their bodies.
David Kipling, who studies ageing at Cardiff University, said: "The goal for human tissue 'rejuvenation' would be to remove senescent cells, or else compensate for the deleterious effects they have on tissues and organs. Although this is a fascinating study, it must be remembered that mice are not little men, particularly with regard to their telomeres, and it remains unclear whether a similar telomerase reactivation in adult humans would lead to the removal of senescent cells."
Lynne Cox, a biochemist at Oxford University, said the study was "extremely important" and "provides proof of principle that short-term treatment to restore telomerase in adults already showing age-related tissue degeneration can rejuvenate aged tissues and restore physiological function."
DePinho said none of Harvard's mice developed cancer after the treatment. The team is now investigating whether it extends the lifespan of mice or enables them to live healthier lives into old age.
Tom Kirkwood, director of the Institute for Ageing and Health at Newcastle University, said: "The key question is what might this mean for human therapies against age-related diseases? While there is some evidence that telomere erosion contributes to age-associated human pathology, it is surely not the only, or even dominant, cause, as it appears to be in mice engineered to lack telomerase. Furthermore, there is the ever-present anxiety that telomerase reactivation is a hallmark of most human cancers."
[from Korean Times Newspaper]
By Kim Tae-gyu
Staff Reporter
 A team of South Korean scientists on Sunday claimed to have created a "cellular fountain of youth," or a small molecule, which enables human cells to avoid aging and dying.
A team of South Korean scientists on Sunday claimed to have created a "cellular fountain of youth," or a small molecule, which enables human cells to avoid aging and dying. The team, headed by Prof. Kim Tae-kook at the Korea Advanced Institute of Science and Technology, argued the newly-synthesized molecule, named CGK733, can even make cells younger.
The findings were featured by the Britain-based Nature Chemical Biology online early today and will be printed as a cover story in the journal’s offline edition early next month.
"All cells face an inevitable death as they age. On this path, cells became lethargic and in the end stop dividing but we witnessed that CGK733 can block the process," Kim said.
"We also found the synthetic compound can reverse aging, by revitalizing already-lethargic cells. Theoretically, this can give youth to the elderly via rejuvenating cells," the 41-year-old said.
Kim expected that the CGK733-empowered drugs that keep cells youthful far beyond their normal life span would be commercialized in less than 10 years.
Other researchers here heaped praises on the discovery but they were cautious about the practical therapeutic application of the new substance.
"Obviously, it is an innovative finding. But we need to see whether or not CGK733 could really rejuvenate cells inside human bodies without generating side effects," Prof. Kim Sung-hoon at Seoul National University said.
Prof. Kim Tae-kook, however, is confident about the commercial viability of CGK733, believing the efficiency of the material was created using state-of-the-art magnetic nano-probe technology.
"We have the magnet-associated technology to identify molecular targets inside living cells, which allowed us to examine the mechanisms of CGK733 directly," Kim said.
"Unlike other research teams that must make candidates materials for drugs without being able to see their intra-cell activities, we know the precise mechanism of CGK733. So we have the better chance of making a success of the substance," he continued.
Indeed, Kim basked in global recognition last June when he and his associates developed a technology dubbed MAGIC, short for magnetism-based interactive capture.
MAGIC uses fluorescent materials to check whether any drug can mix with targeted proteins inside the cell. The results were globally recognized by being printed by the U.S.-based journal Science at the time.
"MAGIC is kind of a source technology to see inside cells. Based on the method, we also found a pair of promising substances that can deal with cancers," Kim said.
ScienceDaily (June 16, 2009) -- Researchers at The Wistar Institute have defined a key target of an evolutionarily conserved protein that regulates the process of aging. The study, published June 11 in Nature, provides fundamental knowledge about key mechanisms of aging that could point toward new anti-aging strategies and cancer therapies.
Scientists have long known that a class of proteins called sirtuins promotes fitness and longevity in most organisms ranging from single-celled yeast to mammals. At the cellular level, sirtuins protect genome integrity, enhance resistance to adverse stresses, and antagonize senescence. However, the underlying molecular mechanisms have remained poorly understood.
The team, led by senior author Shelley Berger, Ph.D., Hilary Koprowski Professor at The Wistar Institute, demonstrated for the first time a molecular target for a member of this class, Sir2, in regulation of aging in yeast cells. Sir2 removes an acetyl group attached to a specific site (lysine at position 16 or K16) on histone H4óhistones are proteins that package and organize the long strands of DNA within the nucleus and also are central regulators in turning genes on and off. The study reveals that removal of this acetyl group by Sir2 near the chromosome ends -- the telomeres -- is important for yeast cells to maintain the ability to replicate. Researchers found that Sir2 levels decline as cells age, and there is a concomitant accumulation of the acetylation mark along with disrupted histone organization at telomeres.
Deacetylation of H4K16 by Sir2 and consequent telomere stability play a major role in maintaining long lifespan in yeast. Since sirtuins deacetylate many different proteins, these results clarify a key role of Sir2 protein in control of lifespan.
"Some modifications on histones, like this acetylation on histone H4 lysine 16, are persistent and are maintained through generations of cell divisions. This DNA-independent inheritance is called epigenetics," Berger says. "Characteristic epigenetic features have been discovered for various developmental processes in recent years. Understanding epigenetic changes associated with aging is a hugely exciting direction in aging research. It will provide insights and ideas not only for new therapies to regulate cells that have lost control of proliferation, such as 'immortal' cells found in cancers, but also for new strategies to maintain health and fitness."
"We plan to continue to search for new targets of Sir2 and other aging regulators," says lead author Weiwei Dang, Ph.D., a postdoctoral scientist working with Berger. "We are designing unbiased screens for other aging targets and mechanisms in chromatin. Using yeast as our aging model enables us to do many discovery screens that are impossible with other, more complex organisms. Yet it is remarkable that many of these chromatin mechanisms associated with yeast could turn out to be relevant even for aging human cells."
Handle With Care: Telomeres Resemble DNA Fragile Sites
ScienceDaily (July 17, 2009) -- Telomeres, the repetitive sequences of DNA at the ends of linear chromosomes, have an important function: They protect vulnerable chromosome ends from molecular attack. Researchers at Rockefeller University now show that telomeres have their own weakness. They resemble unstable parts of the genome called fragile sites where DNA replication can stall and go awry. But what keeps our fragile telomeres from falling apart is a protein that ensures the smooth progression of DNA replication to the end of a chromosome.
The research, led by Titia de Lange, head of the Laboratory of Cell Biology and Genetics, and first author Agnel Sfeir, a postdoctoral associate in the lab, suggests a striking similarity between telomeres and common fragile sites, parts of the genome where breaks tend to occur, albeit infrequently. (Humans have 80 common fragile sites, many of which have been linked to cancer.) De Lange and Sfeir found that these newly discovered fragile sites make it difficult for DNA replication to proceed, a discovery that unveils a new replication problem posed by telomeres.
At the center of the discovery is a protein known as TRF1, which de Lange, in an effort to understand how telomeres protect chromosome ends, discovered in 1995. Using a conditional mouse knockout, de Lange and Sfeir have now revealed that TRF1, which is part of a six-protein complex called shelterin, enables DNA replication to drive smoothly through telomeres with the aid of two other proteins.
"Telomeric DNA has a repetitive sequence that can form unusual DNA structures when the DNA is unwound during DNA replication," says de Lange. "Our data suggest that TRF1 brings in two proteins that can take out these structures in the telomeric DNA. In other words, TRF1 and its helpers remove the bumps in the road so that the replication fork can drive through."
The work, published in the July 10 issue of Cell, began when Sfeir deleted TRF1 and saw that the telomeres resembled common fragile sites, suggesting that TRF1 protects telomeres from becoming fragile. Instead of a continuous string of DNA, the telomeres were broken into fragments of twos and threes. To see if the replication fork stalls at telomeres, de Lange and Sfeir joined forces with Carl L. Schildkraut, a researcher at Albert Einstein College of Medicine in New York City. Using a technique called SMARD, the researchers observed the dynamics of replication across individual DNA molecules -- the first time this technique has been used to study telomeres. In the absence of TRF1, the fork often stalled for a considerable amount of time.
The only other known replication problem posed by telomeres was solved in 1985 when it was shown that the enzyme telomerase elongates telomeres, which shorten during every cell division. The second problem posed by telomeres, the so-called end-protection problem, was solved by de Lange and her colleagues when they found that shelterin protects the ends of linear chromosomes, which look like damaged DNA, from unnecessary repair. Working with TRF1, the very first shelterin protein ever to be identified, de Lange and Sfeir have not only unveiled a completely unanticipated replication problem at telomeres, they have also shown how it is solved.
The research lays new groundwork for the study of common fragile sites throughout the genome, explains de Lange. "Fragile sites have always been hard to study because no specific DNA sequence preceeds or follows them," she says. "In contrast, telomeres represent fragile sites with a known sequence, which may help us understand how common fragile sites break throughout the genome -- and why."
Also see Blood Cancer Stopped By Shortening Chromosomes
From ViewZone @ http://www.viewzone.com/aging.html
For further enlightening information enter a word or phrase into the search box @ New Illuminati or click on any label/tag at the bottom of the page @ http://nexusilluminati.blogspot.com
And see
The Her(m)etic Hermit - http://hermetic.blog.com
New Illuminati – http://nexusilluminati.blogspot.com
New Illuminati on Facebook - http://www.facebook.com/pages/New-Illuminati/320674219559
This material is published under Creative Commons Fair Use Copyright (unless an individual item is declared otherwise by copyright holder) – reproduction for non-profit use is permitted & encouraged, if you give attribution to the work & author - and please include a (preferably active) link to the original along with this notice. Feel free to make non-commercial hard (printed) or software copies or mirror sites - you never know how long something will stay glued to the web – but remember attribution! If you like what you see, please send a tiny donation or leave a comment – and thanks for reading this far…
From the New Illuminati – http://nexusilluminati.blogspot.com
Anti Aging or Age Reversal would be a disaster for the human race. As only a select few would have access to it and benefit from it. The Trillionaires or Secret Rich would make sure only they could benefit, or those they deem beneficial to them. So you would have the 1% super rich and super powerful getting even more powerful at the cost of the rest of us. Do you really think an anti aging product would be made available to the average Joe? The world is already over populated! These people would not allow the rest of us access. And to be honest. I pretty much guarantee if it ever does become a reality, it will be kept secret from the rest of us.
ReplyDeletelet us go forward in being immortals....that we may not to die
ReplyDeletelets find a cure now for this dreadful thing called aging ...
ReplyDelete